Chem 121-2010 Problem Set #7
1. Write Lewis structures for each of the following molecules: a) PH3; b) SCl2; c) HI; d) CH4
2. Write Lewis structures for each of the following molecules or ions and assign formal charges to each atom when applicable:
a) CI4; b) N2O; c) SiH4; d) Cl2CO; e) H3COH; f) CN-; g) BrO-
3. Write a Lewis structure that obeys the octet rule for each of the following molecules or ions. Include resonance structures if necessary and assign formal charges to each atom.
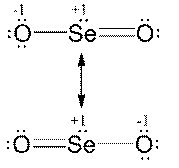
a) SeO2; b) NO3-; c) ClO-; d) NO2-
4. How important is the following resonance structure, does not include formal charges, to the overall structure of carbon dioxide? Explain.

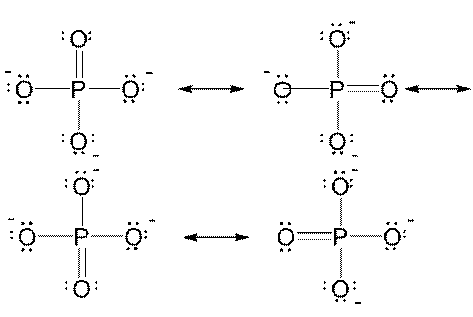
5. Write Lewis structures for each of the following ions. Include resonance structures if necessary and assign formal charges to all atoms. If necessary, expand the octet of the central atom to lower formal charge. a) PO43-; b) CN-; c) SO32-; d) ClO2-
6. Write Lewis structures for each of the following species. Use expanded octets as necessary.
a) PF5; b) I3-; c) SF4; d) GeF4
7. Determine the molecular geometry and make a sketch of each of the following molecules or ions. a) SF4; b) ClF3; c) IF2-; d) IBr4-
8. The valence electron configurations of several atoms are shown below. How many bonds can each atom make without hybridization?
a) Be: 2s2; b) P: 3s23p3; c) F: 2s22p5
9. Identify the valence electrons, without hybridization, involved for all the atoms in PH3. What bond angle do you expect from the unhybridized orbitals? How well does this bond angle agree with the experimentally determined value of 93.3°?
10. Write a hybridization scheme for each of the following molecules or ions.
a) COCl2(carbon is the central atom); b) BrF5; c) XeF2; d) I3-
11. Determine whether a bond between each of the following pairs of atoms would be nonpolar covalent, polar covalent, or ionic. a) Br and Br; b) C and Cl; c) C and S; d) Sr and O
12. Determine whether each of the following molecules is polar or nonpolar.
a) PF3; b) SBr2; c) CHCl3; d) CS2
13. Determine the kind of intermolecular forces that are present in each of the following elements or compounds: a) Kr; b) NCl3; c) SiH4; d) HF; e) N2; f) NH3; g) CO; h) CCl4
Answer Set for Chem 121-2010 Problem Set #7:
|
1.a)
|
b)
|
|
c)
|
d)
|
|
2.a)
|
b)
|
|
c)
|
d)
|
|
e)
|
f)
|
|
g)
|
|
|
3.a)
|
b)
|
|
c)
|
d)
|
4. The resonance structure does not provide a significant contribution to the resonance hybrid as it has a +1 formal charge on a very electronegative oxygen.
|
5.a)
|
|
b)
|
|
c)
|
|
d)
|
|
6.a)
|
b)
|
|
c)
|
d)
|
|
7.a) seesaw
|
b) T-shaped
|
|
c) linear
|
d) square planar
|
8.a) 0; b) 3; c) 1
9. For P: 3px1 3py1 3pz1, H: 1s1. Expected bond angle is 90.0° which is very close to the experimental value.
10.a) sp2; b) sp3d2; c) sp3d; d) sp3d
11.a) nonpolar covalent; b) polar covalent; c) nonpolar covalent; d) ionic bond
12.a) polar; b) nonpolar; c) polar; d) nonpolar
13.a) dispersion; b) dispersion, dipole-dipole; c) dispersion; d) dispersion, dipole-dipole, hydrogen bonding; e) dispersion; f) dispersion, dipole-dipole, hydrogen bonding; g) dispersion, dipole-dipole; h) dispersion